Guanidines from 'toxic substances' to compounds with multiple biological applications – Detailed outlook on synthetic procedures employed for the synthesis of guanidines - ScienceDirect

Guanidines from 'toxic substances' to compounds with multiple biological applications – Detailed outlook on synthetic procedures employed for the synthesis of guanidines - ScienceDirect
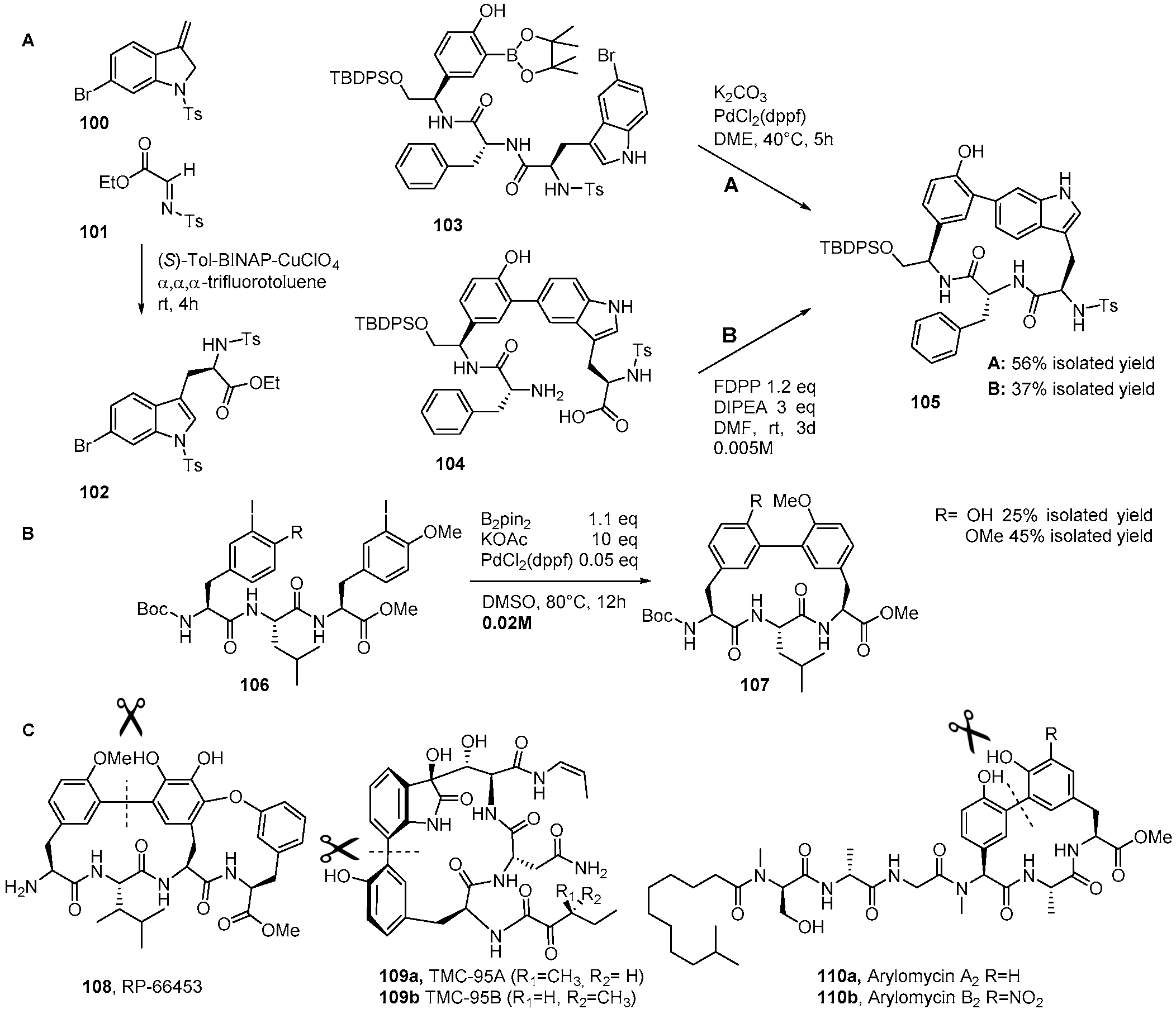
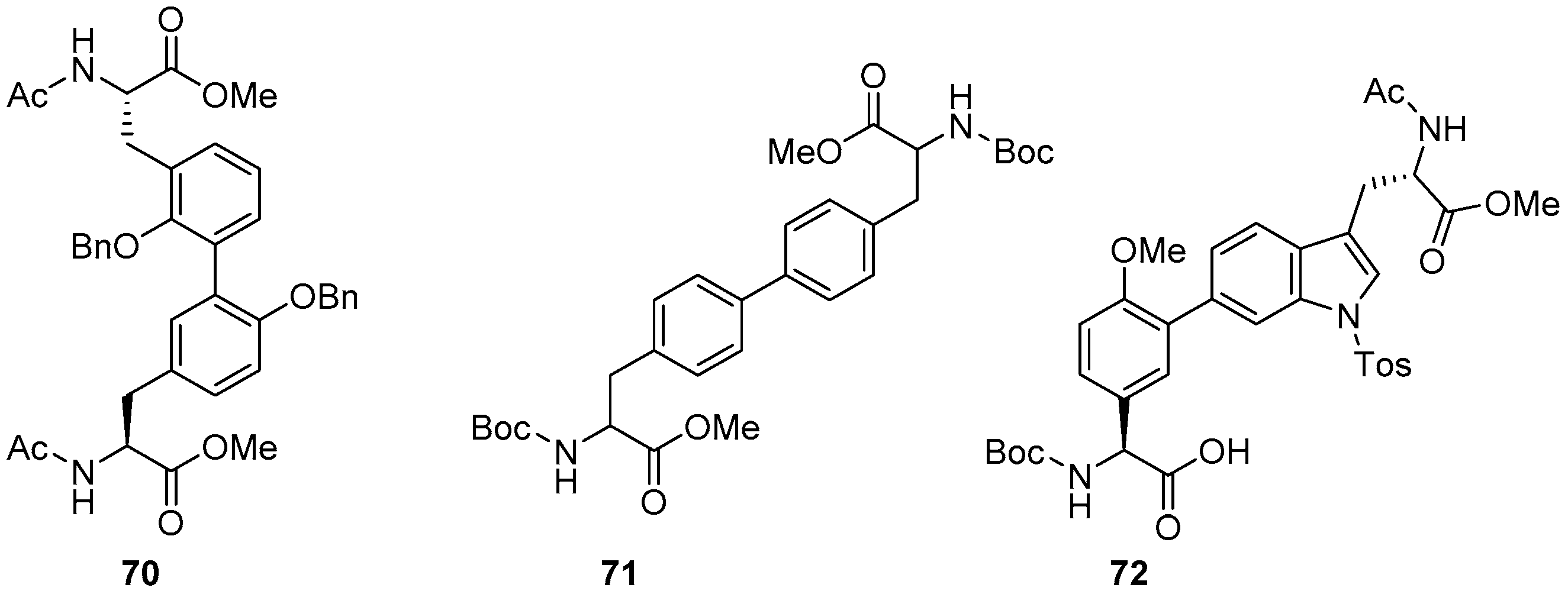
Catalysts | Free Full-Text | The Suzuki–Miyaura Cross-Coupling as a Versatile Tool for Peptide Diversification and Cyclization | HTML
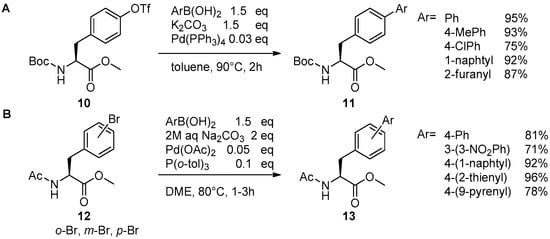
Catalysts | Free Full-Text | The Suzuki–Miyaura Cross-Coupling as a Versatile Tool for Peptide Diversification and Cyclization | HTML
Guanidine nitrate (52470-25-4, 506-93-4) - Chemical Safety, Models, Suppliers, Regulation, and Patents - Chemchart

Guanidines from 'toxic substances' to compounds with multiple biological applications – Detailed outlook on synthetic procedures employed for the synthesis of guanidines - ScienceDirect

Catalysts | Free Full-Text | The Suzuki–Miyaura Cross-Coupling as a Versatile Tool for Peptide Diversification and Cyclization | HTML
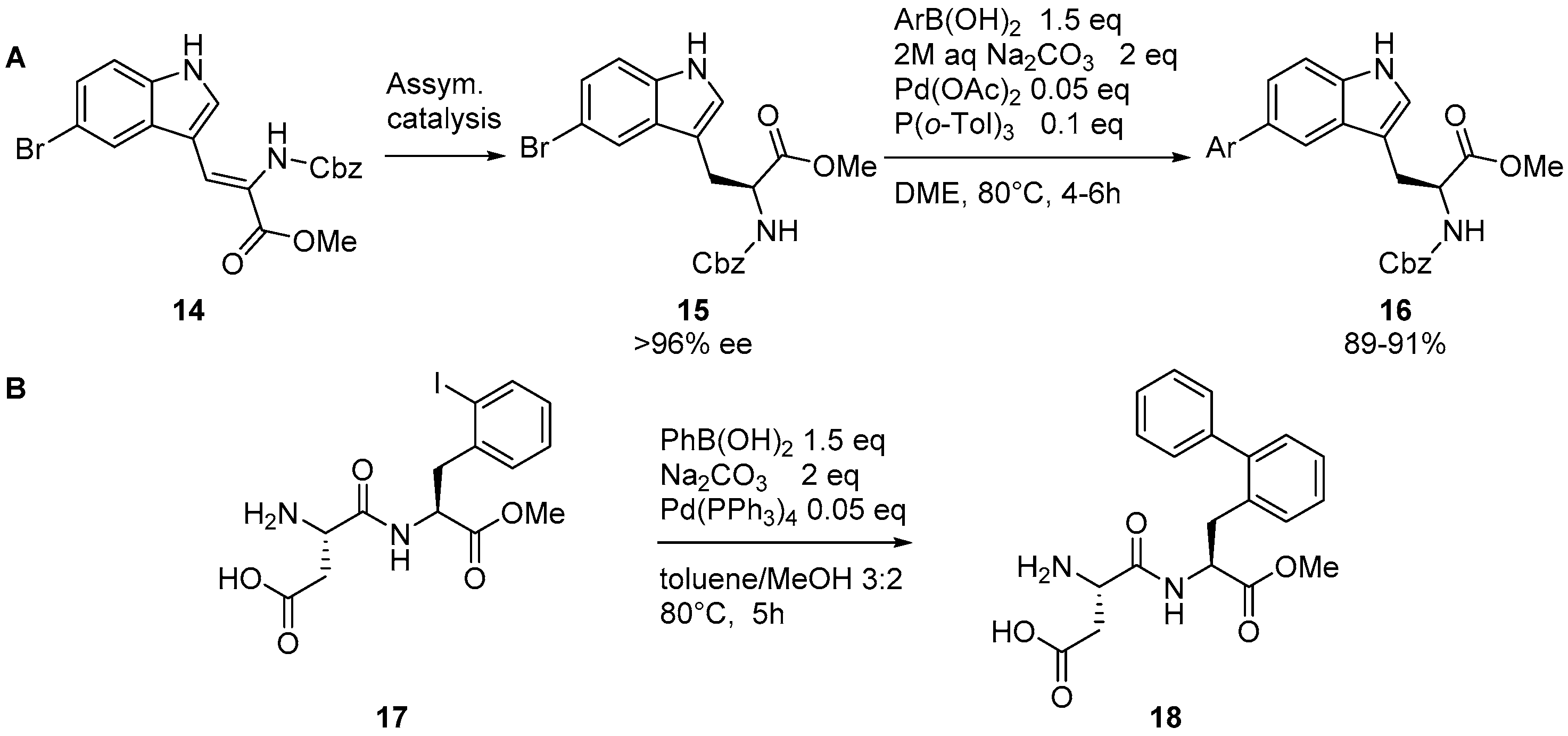
Catalysts | Free Full-Text | The Suzuki–Miyaura Cross-Coupling as a Versatile Tool for Peptide Diversification and Cyclization | HTML
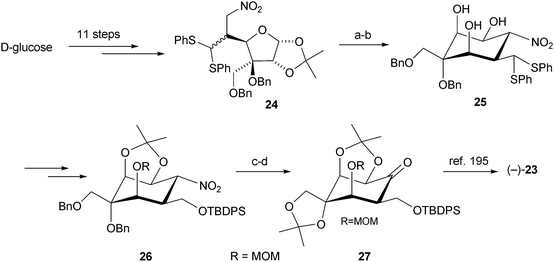
The chemistry and biology of organic guanidine derivatives - Natural Product Reports (RSC Publishing)
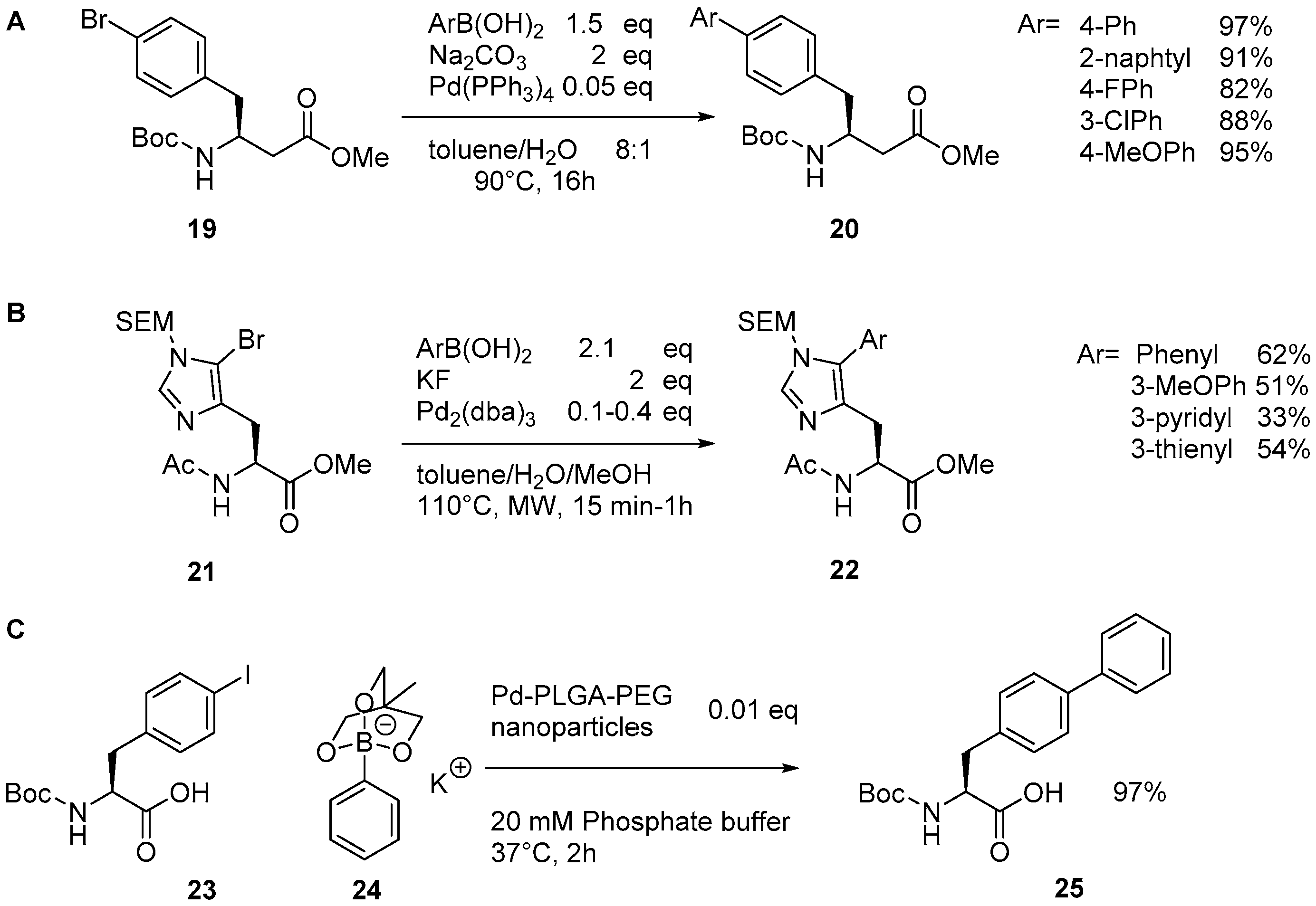
Catalysts | Free Full-Text | The Suzuki–Miyaura Cross-Coupling as a Versatile Tool for Peptide Diversification and Cyclization | HTML
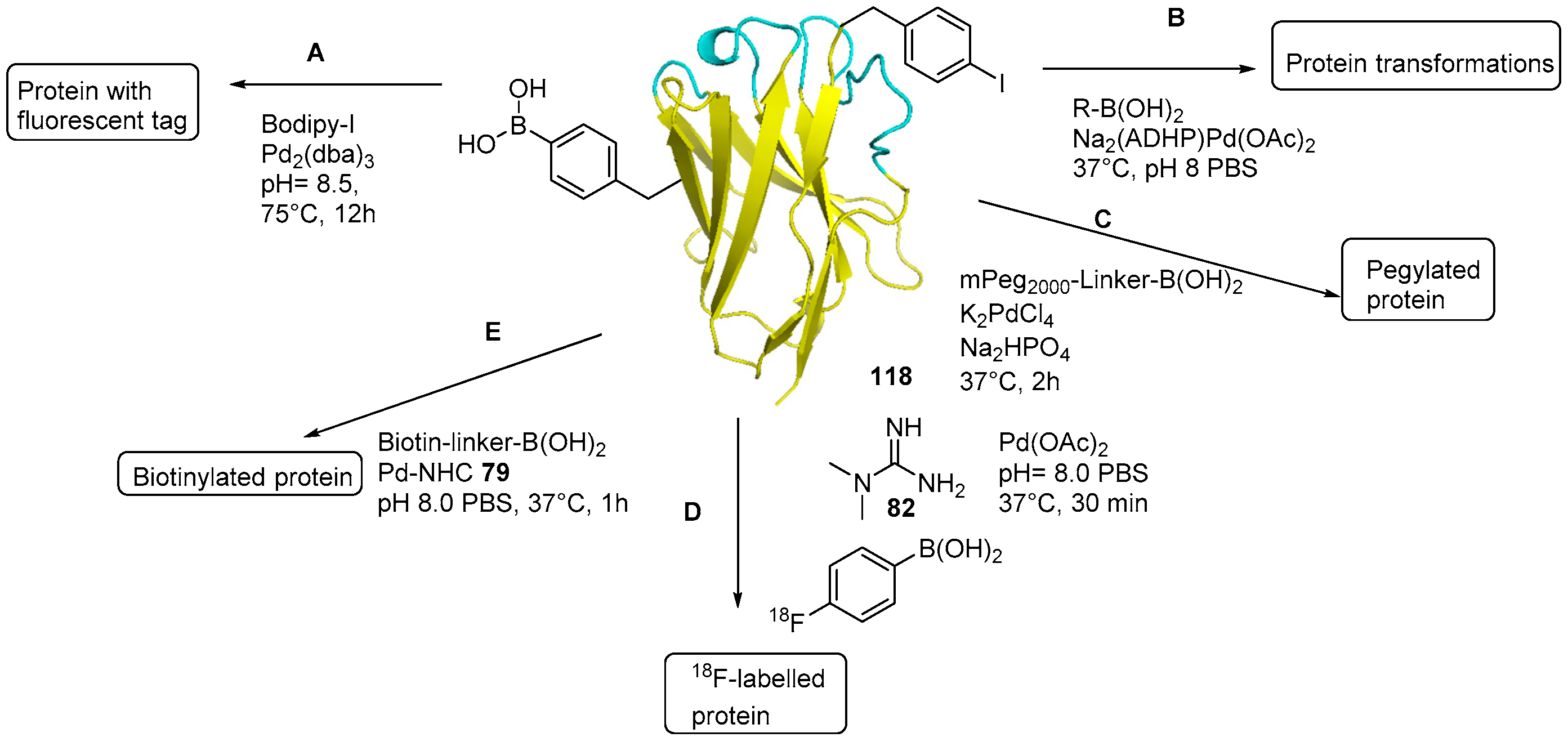
Catalysts | Free Full-Text | The Suzuki–Miyaura Cross-Coupling as a Versatile Tool for Peptide Diversification and Cyclization | HTML

Palladium catalyzed P–C coupling – a powerful tool for the syntheses of hydrophilic phosphines - ScienceDirect

Guanidines from 'toxic substances' to compounds with multiple biological applications – Detailed outlook on synthetic procedures employed for the synthesis of guanidines - ScienceDirect
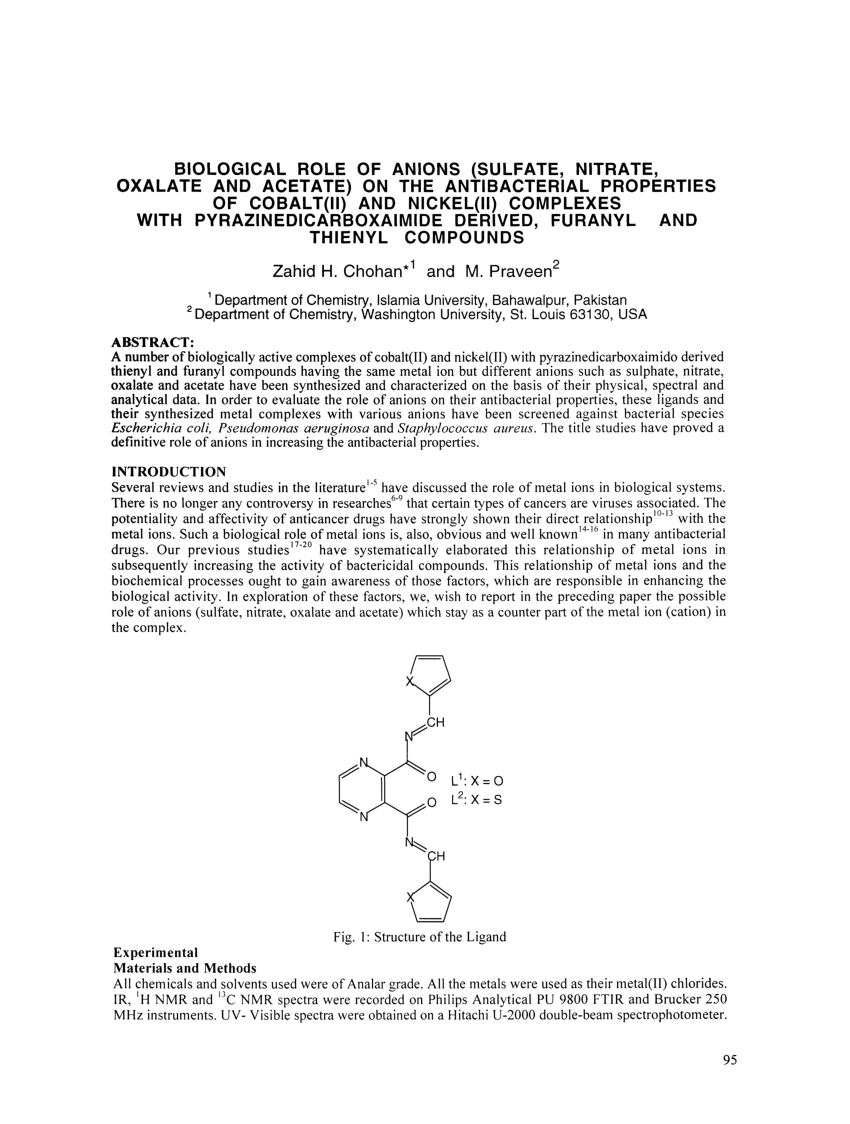
PDF) Biological Role of Anions (Sulfate, Nitrate , Oxalate and Acetate) on the Antibacterial Properties of Cobalt (II) and Nickel(II) Complexes With Pyrazinedicarboxaimide Derived, Furanyl and Thienyl Compounds
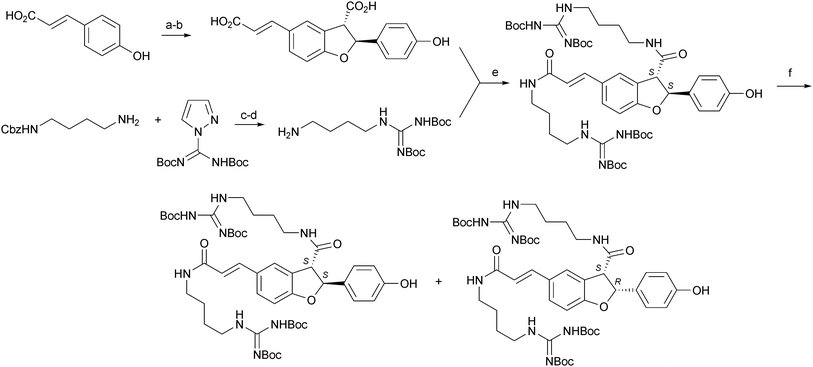
The chemistry and biology of organic guanidine derivatives - Natural Product Reports (RSC Publishing)

Palladium catalyzed P–C coupling – a powerful tool for the syntheses of hydrophilic phosphines - ScienceDirect

Histone acetyltransferase inhibitors: An overview in synthesis, structure-activity relationship and molecular mechanism - ScienceDirect

Catalysts | Free Full-Text | The Suzuki–Miyaura Cross-Coupling as a Versatile Tool for Peptide Diversification and Cyclization | HTML