PDF) Synthesis and Structural Analysis of Palladium(II) Complexes Containing Neutral or Anionic C 2 -Symmetric Bis(oxazoline) Ligands: Effects of Substituents in the 5-Position

PDF) Synthesis of palladium complexes with bis(diphenylphosphinomethyl)amino ligands: A catalyst for the Heck reaction of aryl halide with methyl acrylate | Osman Serindag - Academia.edu

Structures of isolated palladacycles 2-5, palladium precursors 6,7 and... | Download Scientific Diagram
![Synthesis of the diphenylacetylene-based, tetra-amine ligand 1,2-bis(3,5-bis [(dimethylamino)methyl]phenyl)acetylene by palladium-catalysed cross-coupling: isolation and crystal structure of the catalyst trans-(3,5- bis[(dimethylamino)methyl]phenyl)bis ... Synthesis of the diphenylacetylene-based, tetra-amine ligand 1,2-bis(3,5-bis [(dimethylamino)methyl]phenyl)acetylene by palladium-catalysed cross-coupling: isolation and crystal structure of the catalyst trans-(3,5- bis[(dimethylamino)methyl]phenyl)bis ...](https://ars.els-cdn.com/content/image/1-s2.0-S0022328X99006658-sc1.gif)
Synthesis of the diphenylacetylene-based, tetra-amine ligand 1,2-bis(3,5-bis [(dimethylamino)methyl]phenyl)acetylene by palladium-catalysed cross-coupling: isolation and crystal structure of the catalyst trans-(3,5- bis[(dimethylamino)methyl]phenyl)bis ...

palladium(II)%C2%A0chloride.jpg)


_chlori[804174_Bispalladium(II)_chlori-ALL].jpg)

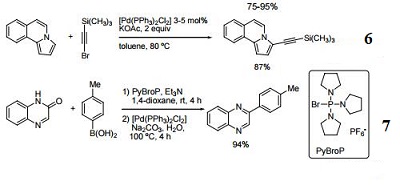



![Di μ furan 2 selenolato bis[(furan 2 selenolato)(triphenylphosphine) palladium(II)] Di μ furan 2 selenolato bis[(furan 2 selenolato)(triphenylphosphine) palladium(II)]](https://data01.123dok.com/thumb/q0/58/2mgy/Pki4ZiPT5VIqTD1so/cover.webp)