
Palladium N-heterocyclic carbene catalyzed regioselective thiolation of 1-aryl-3-methyl-1H-pyrazol-5(4H)-ones using aryl thiols - ScienceDirect

Molecules | Free Full-Text | Macrocyclic Drugs and Synthetic Methodologies toward Macrocycles | HTML

Palladium/N-heterocyclic carbene catalysed regio and diastereoselective reaction of ketones with allyl reagents via inner-sphere mechanism | Nature Communications

Recent advances in the Suzuki–Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media - Green Chemistry (RSC Publishing) DOI:10.1039/C8GC02860E

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium N-heterocyclic carbene catalyzed regioselective thiolation of 1-aryl-3-methyl-1H-pyrazol-5(4H)-ones using aryl thiols - ScienceDirect
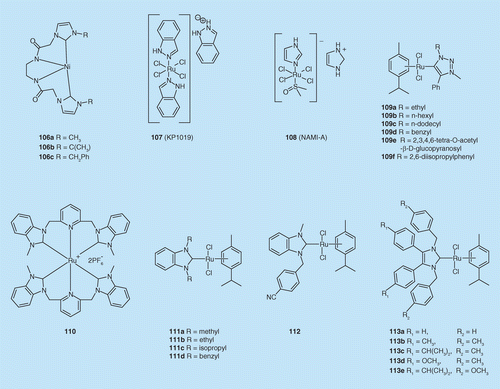
N-heterocyclic carbene metal complexes as bio-organometallic antimicrobial and anticancer drugs | Future Medicinal Chemistry

Palladium N-heterocyclic carbene catalyzed regioselective thiolation of 1-aryl-3-methyl-1H-pyrazol-5(4H)-ones using aryl thiols - ScienceDirect

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

An Antitumor Bis(N-Heterocyclic Carbene)Platinum(II) Complex That Engages Asparagine Synthetase as an Anticancer Target. - Angew. Chem. Int. Ed. - X-MOL

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

PDF) Ultra‐small sugar‐substituted N‐heterocyclic carbene‐protected palladium nanoparticles and catalytic activity

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Recent advances in the catalytic applications of GO/rGO for green organic synthesis in: Green Processing and Synthesis Volume 9 Issue 1 (2020)

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -
New trends in the functionalization of metallic gold: from organosulfur ligands to N-heterocyclic carbenes

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -

Robust gold nanorods stabilized by bidentate N-heterocyclic-carbene–thiolate ligands | Nature Chemistry
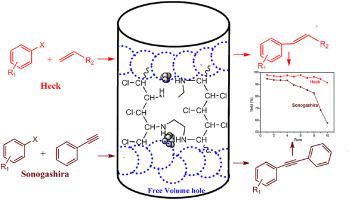
Encapsulating palladium nanoparticles inside ethylenediamine functionalized and crosslinked chlorinated poly(vinyl chloride) nanofibers as an efficient and stable heterogeneous catalyst for coupling reactions - J. Phys. Chem. Solids - X-MOL

Palladium‐Catalyzed C–S Bond Formation: Rate and Mechanism of the Coupling of Aryl or Vinyl Halides with a Thiol Derived from a Cysteine - Moreau - 2005 - European Journal of Organic Chemistry -


