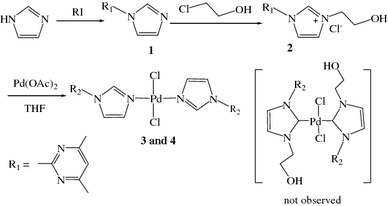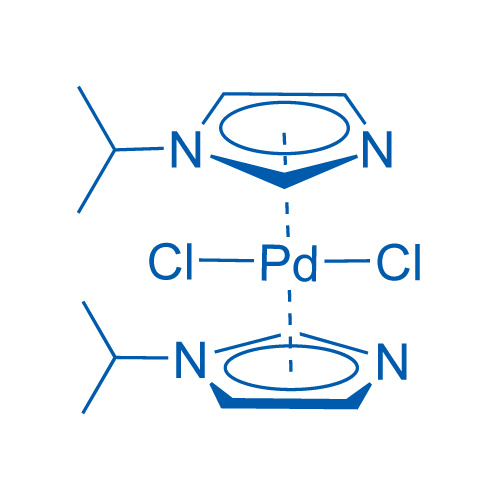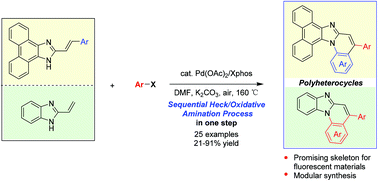
Palladium-catalyzed tandem one-pot synthesis of π-expanded imidazoles through a sequential Heck and oxidative amination reaction - Organic & Biomolecular Chemistry (RSC Publishing)

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds
![PDF) Mixed Ligand, Palladium(II) and Platinum(II) Complexes of Tertiary Diphosphines with S-1H Benzo[D] Imidazole-2-Yl Benzothioate PDF) Mixed Ligand, Palladium(II) and Platinum(II) Complexes of Tertiary Diphosphines with S-1H Benzo[D] Imidazole-2-Yl Benzothioate](https://i1.rgstatic.net/publication/316589515_Mixed_Ligand_PalladiumII_and_PlatinumII_Complexes_of_Tertiary_Diphosphines_with_S-1H_BenzoD_Imidazole-2-Yl_Benzothioate/links/5a1e819faca272cbfbc04f0f/largepreview.png)
PDF) Mixed Ligand, Palladium(II) and Platinum(II) Complexes of Tertiary Diphosphines with S-1H Benzo[D] Imidazole-2-Yl Benzothioate
![NHC-palladium chloride-imidazole[NHC-Pd(II)-Im]complexes|1314876-23-7——Nanjing Finetech Chemical Co., Ltd. NHC-palladium chloride-imidazole[NHC-Pd(II)-Im]complexes|1314876-23-7——Nanjing Finetech Chemical Co., Ltd.](http://www.fine-chemtech.com/Uploads/5837a4c45b105.png)
NHC-palladium chloride-imidazole[NHC-Pd(II)-Im]complexes|1314876-23-7——Nanjing Finetech Chemical Co., Ltd.

C-H bonds as ubiquitous functionality: a general approach to complex arylated imidazoles via regioselective sequential arylation of all three C-H bonds and regioselective N-alkylation enabled by SEM-group transposition. - Abstract - Europe
![C(sp2)-H Functionalization of Imidazole at the C2- and C4-Position via Palladium-Catalyzed Isocyanide Insertion Leading to Indeno[1,2-d]imidazole and Imidazo[1,2-a]indole Derivatives.,The Journal of Organic Chemistry - X-MOL C(sp2)-H Functionalization of Imidazole at the C2- and C4-Position via Palladium-Catalyzed Isocyanide Insertion Leading to Indeno[1,2-d]imidazole and Imidazo[1,2-a]indole Derivatives.,The Journal of Organic Chemistry - X-MOL](https://xpic.x-mol.com/20200822%2F10.1021_acs.joc.0c01454.gif)
C(sp2)-H Functionalization of Imidazole at the C2- and C4-Position via Palladium-Catalyzed Isocyanide Insertion Leading to Indeno[1,2-d]imidazole and Imidazo[1,2-a]indole Derivatives.,The Journal of Organic Chemistry - X-MOL

Polymer-supported palladium-imidazole complex catalyst for hydrogenation of substituted benzylideneanilines - ScienceDirect
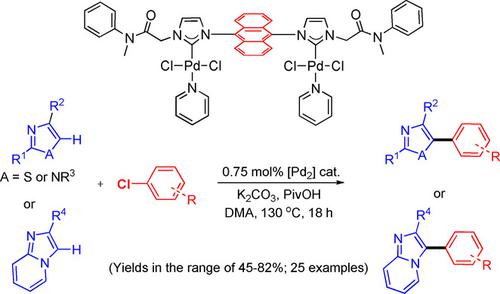
Dimetallic Palladium‐NHC Complexes: Synthesis, Characterization, and Catalytic Application for Direct C−H Arylation Reaction of Heteroaromatics with Aryl Chlorides - Adv. Synth. Catal. - X-MOL

Polymer-supported palladium-imidazole complex catalyst for hydrogenation of substituted benzylideneanilines - ScienceDirect

Air-stable imidazole-imine palladium complexes for Suzuki–Miyaura coupling: Toward an efficient, green synthesis of biaryl compounds - ScienceDirect
![884879-23-6 | PHENYLALLYLCHLORO-[1,3-BIS(DIISOPROPYLPHENYL)IMIDAZOLE -2-YLIDENE]PALLADIUM(II) | Hangzhou Keying Chem Co., Ltd. 884879-23-6 | PHENYLALLYLCHLORO-[1,3-BIS(DIISOPROPYLPHENYL)IMIDAZOLE -2-YLIDENE]PALLADIUM(II) | Hangzhou Keying Chem Co., Ltd.](https://qncdn.chemcd.cn/keying/structure/KY155655.png)
884879-23-6 | PHENYLALLYLCHLORO-[1,3-BIS(DIISOPROPYLPHENYL)IMIDAZOLE -2-YLIDENE]PALLADIUM(II) | Hangzhou Keying Chem Co., Ltd.

Completely N1-selective palladium-catalyzed arylation of unsymmetric imidazoles: application to the synthesis of nilotinib. - Abstract - Europe PMC

Structure, dynamics and catalytic activity of palladium(II) complexes with imidazole ligands - ScienceDirect
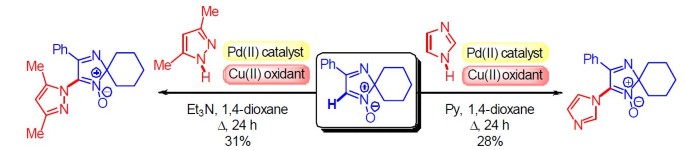
The First Example of Palladium(II)-Catalyzed Oxidative C–N Cross Coupling of 2 H -Imidazole 1-Oxide with Azoles | SpringerLink

Air-stable imidazole-imine palladium complexes for Suzuki–Miyaura coupling: Toward an efficient, green synthesis of biaryl compounds - ScienceDirect
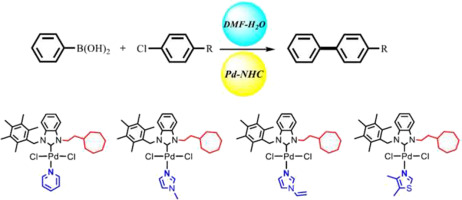
Cycloheptyl substituted N-heterocyclic carbene PEPPSI-type palladium complexes with different N-coordinated ligands: Involvement in Suzuki-Miyaura reaction - J. Organomet. Chem. - X-MOL
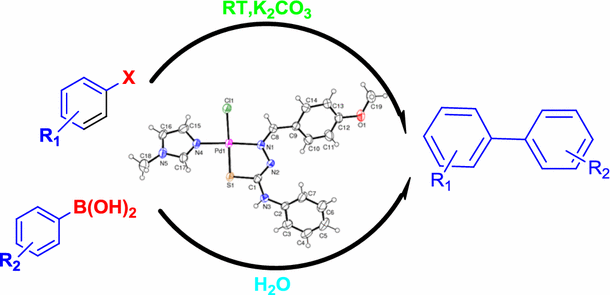
A thiosemicarbazone–palladium(II)–imidazole complex as an efficient pre-catalyst for Suzuki–Miyaura cross-coupling reactions at room temperature in aqueous media | SpringerLink

Mechanistic Studies on the Palladium‐Catalyzed Direct C‐5 Arylation of Imidazoles: The Fundamental Role of the Azole as a Ligand for Palladium - Perego - 2016 - Advanced Synthesis & Catalysis - Wiley Online Library
Palladium(2+) (3-ethenidyl-1,1,3,3-tetramethyldisiloxanyl)ethenide - 1,3-dimesityl-2,3-dihydro-1H-imidazole (1:1:1) | C29H42N2OPdSi2 | ChemSpider