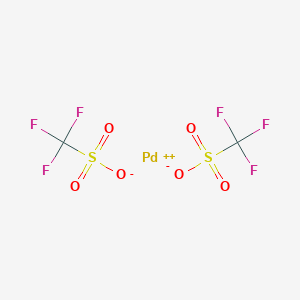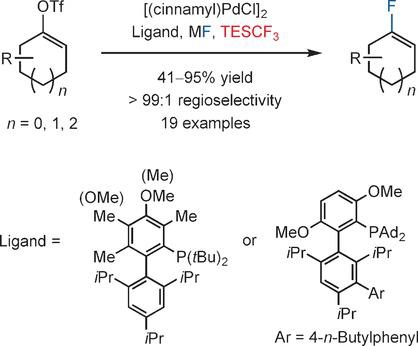
Palladium‐Catalyzed Fluorination of Cyclic Vinyl Triflates: Effect of TESCF3 as an Additive - Angew. Chem. Int. Ed. - X-MOL

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds
![Palladium‐Catalyzed Trifluoroethylation of Benzo[h]quinoline Derivatives by Mesityl(2,2,2‐trifluoroethyl)iodonium Triflate - Asian J. Org. Chem. - X-MOL Palladium‐Catalyzed Trifluoroethylation of Benzo[h]quinoline Derivatives by Mesityl(2,2,2‐trifluoroethyl)iodonium Triflate - Asian J. Org. Chem. - X-MOL](https://xpic.x-mol.com/20190213%2F10.1002_ajoc.201900030.jpg)
Palladium‐Catalyzed Trifluoroethylation of Benzo[h]quinoline Derivatives by Mesityl(2,2,2‐trifluoroethyl)iodonium Triflate - Asian J. Org. Chem. - X-MOL
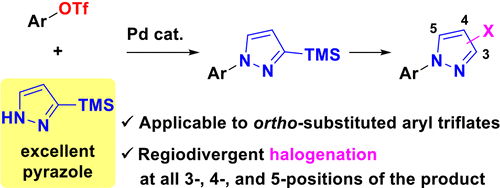
Synthesis of N-Arylpyrazoles by Palladium-Catalyzed Coupling of Aryl Triflates with Pyrazole Derivatives - J. Org. Chem. - X-MOL

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds
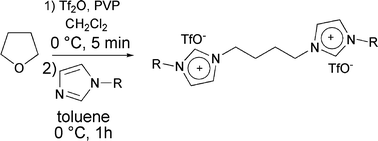
Halide-free highly-pure imidazolium triflate ionic liquids: Preparation and use in palladium-catalysed allylic alkylation - Green Chemistry (RSC Publishing)
![R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl]-diaquo-palladium(II) bis(triflate) | Sigma-Aldrich R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl]-diaquo-palladium(II) bis(triflate) | Sigma-Aldrich](https://www.sigmaaldrich.com/content/dam/sigma-aldrich/structure3/034/a_____705764.eps/_jcr_content/renditions/a_____705764-medium.png)
R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl]-diaquo-palladium(II) bis(triflate) | Sigma-Aldrich
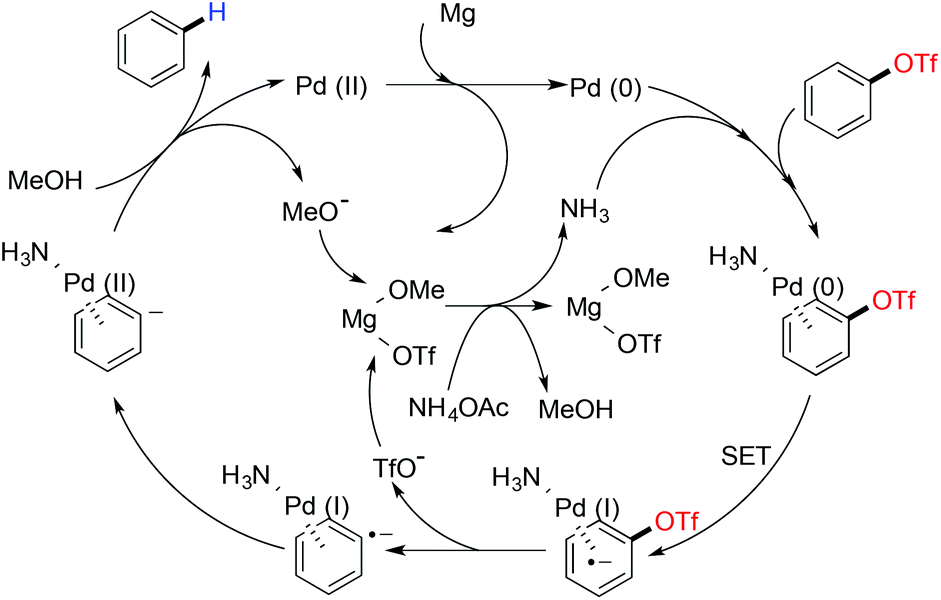
Metal catalyzed defunctionalization reactions - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C5OB01949D

Palladium-catalyzed triethylammonium formate reduction of aryl triflates. A selective method for the deoxygenation of phenols - ScienceDirect

Nucleophilic palladium-catalysed Ar−F bond-forming reaction. a, The... | Download Scientific Diagram
![R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl]-diaquo-palladium(II) bis(triflate) | Sigma-Aldrich R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl]-diaquo-palladium(II) bis(triflate) | Sigma-Aldrich](https://www.sigmaaldrich.com/content/dam/sigma-aldrich/structure3/041/a_____705780.eps/_jcr_content/renditions/a_____705780-medium.png)
R)-(+)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl]-diaquo-palladium(II) bis(triflate) | Sigma-Aldrich

Scheme 1. General reaction for the Suzuki-Miyaura palladium-catalyzed... | Download Scientific Diagram
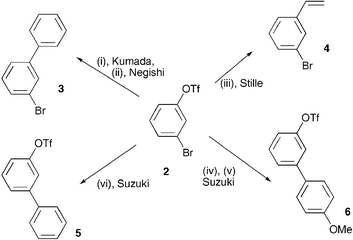

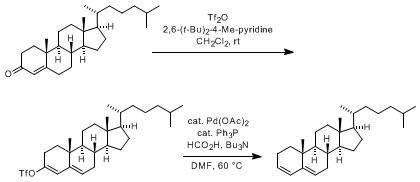


![1,3-Bis(diphenylphosphino)propane]palladium(II) triflate | 137846-38-9 | Sigma-Aldrich 1,3-Bis(diphenylphosphino)propane]palladium(II) triflate | 137846-38-9 | Sigma-Aldrich](https://www.sigmaaldrich.com/content/dam/sigma-aldrich/structure8/037/a_____705772.eps/_jcr_content/renditions/a_____705772-medium.png)