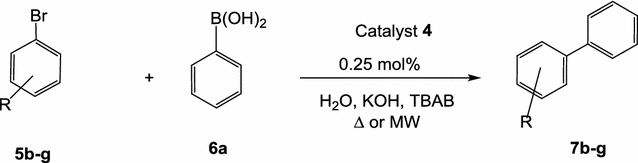
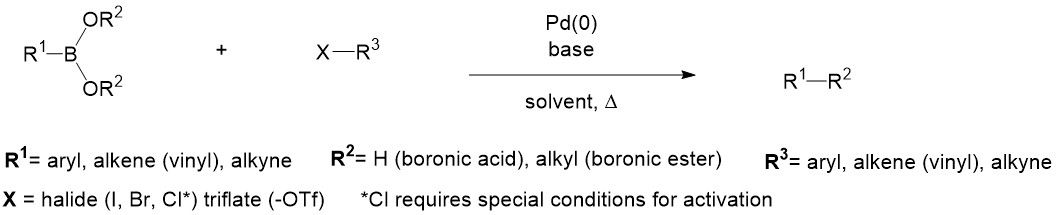
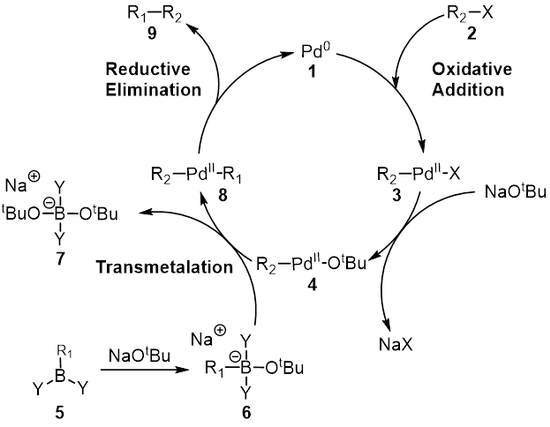
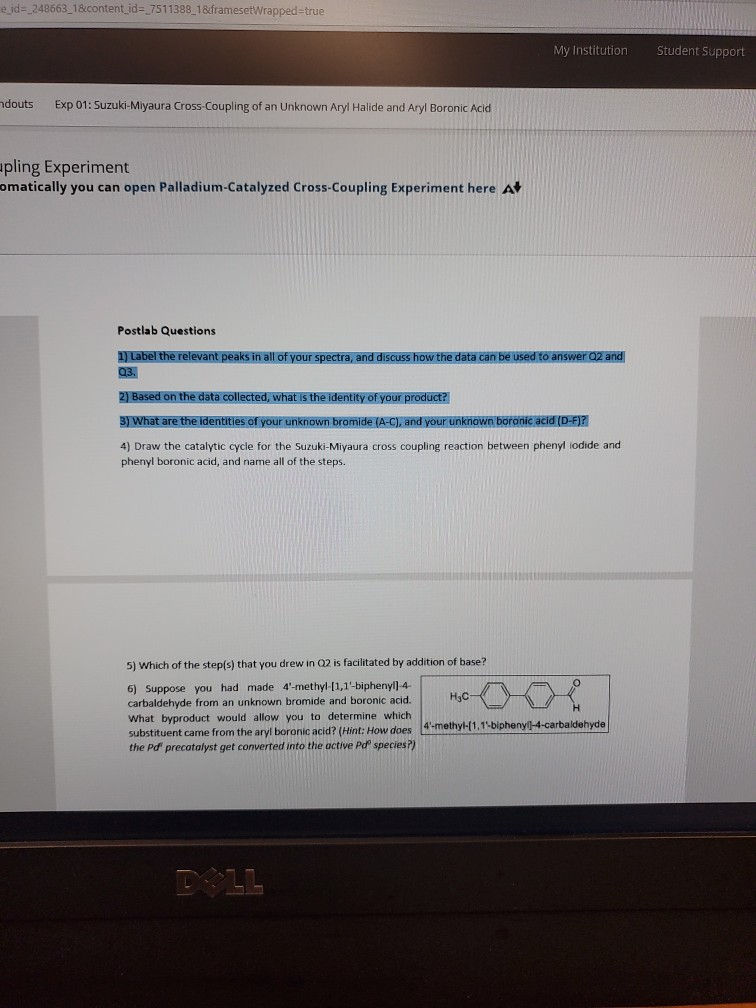
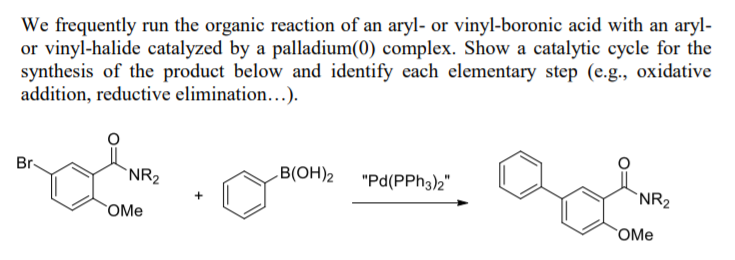
Synthesis of Biaryls via Decarbonylative Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling of Carboxylic Acids - ScienceDirect

Palladium-catalyzed cross-coupling reactions of aryl boronic acids with aryl halides in water - PDF Free Download

Studies on Pd/NiFe 2 O 4 catalyzed ligand-free Suzuki reaction in aqueous phase: synthesis of biaryls, terphenyls and polyaryls – topic of research paper in Chemical sciences. Download scholarly article PDF and
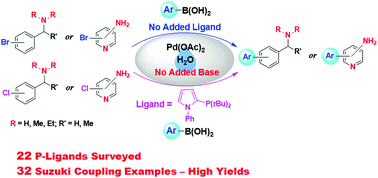
Pd-Catalyzed Suzuki coupling reactions of aryl halides containing basic nitrogen centers with arylboronic acids in water in the absence of added base - New Journal of Chemistry (RSC Publishing)

i>N</i>-Doped porous carbon supported palladium nanoparticles as a highly efficient and recyclable catalyst for the Suzuki coupling reaction
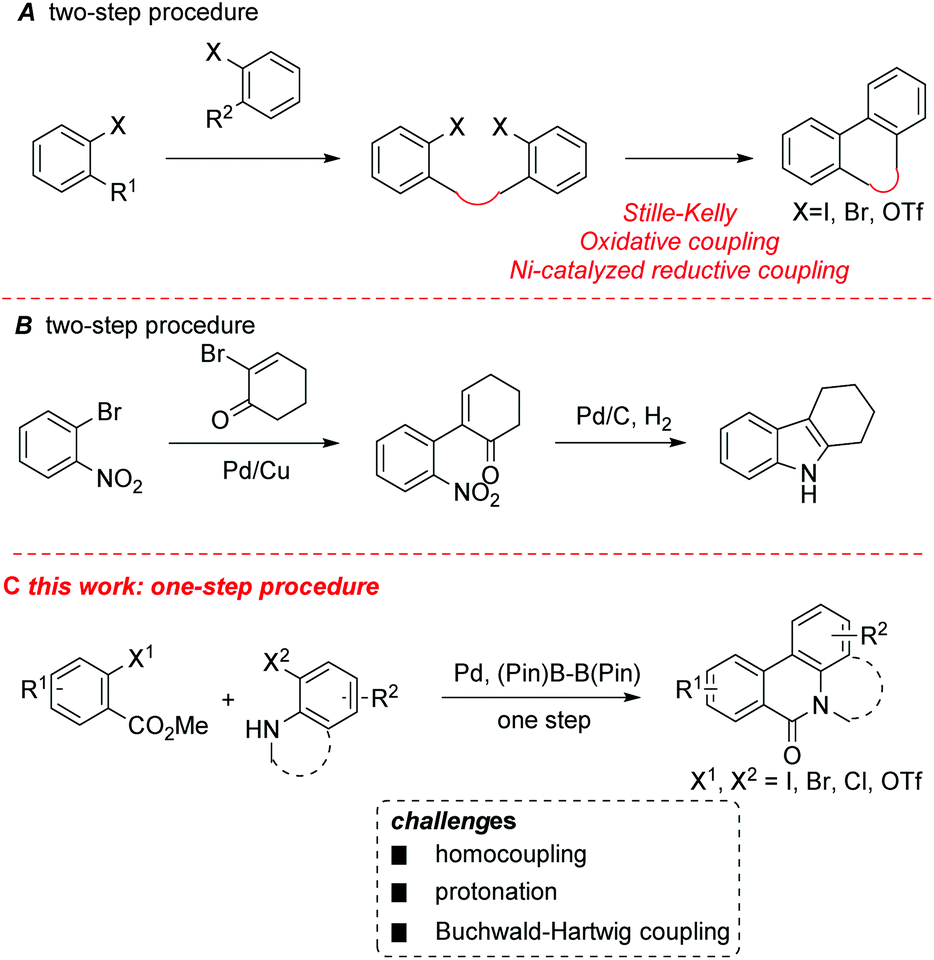
A Pd-catalyzed, boron ester-mediated, reductive cross-coupling of two aryl halides to synthesize tricyclic biaryls - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C7OB01237C

Figure 1 from Palladium-Catalyzed Synthesis of (Hetero)Aryl Alkyl Sulfones from (Hetero)Aryl Boronic Acids, Unactivated Alkyl Halides, and Potassium Metabisulfite. | Semantic Scholar

Suzuki–Miyaura Cross‐Coupling Reactions of Alkylboronic Acid Derivatives or Alkyltrifluoroborates with Aryl, Alkenyl or Alkyl Halides and Triflates - Doucet - 2008 - European Journal of Organic Chemistry - Wiley Online Library
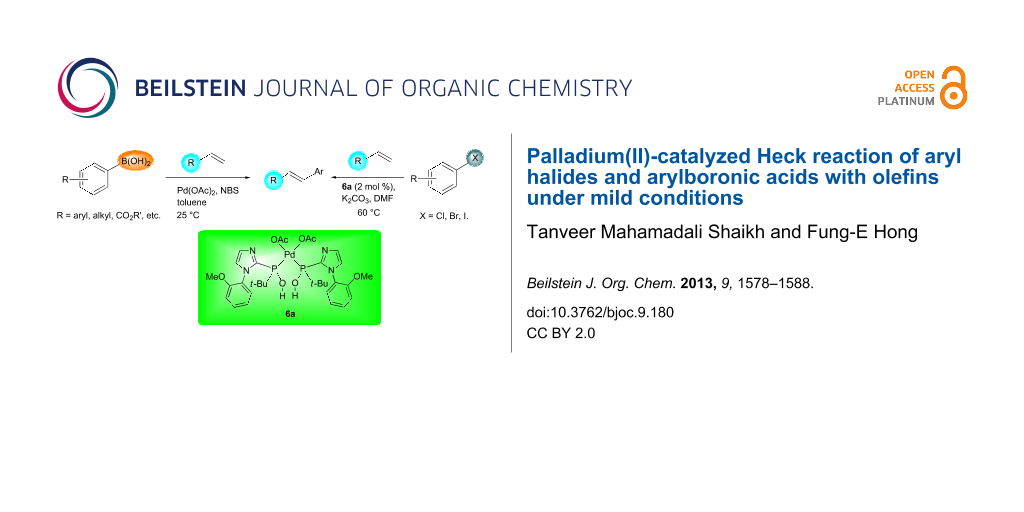
Palladium(II)-catalyzed Heck reaction of aryl halides and arylboronic acids with olefins under mild conditions

PdCl2 Immobilized in Polyacrylamide: a Low Cost and Eco-Friendly Catalyst for Suzuki-Miyaura Reactions

Suzuki-Miyaura cross-coupling of phenylboronic acid with aryl halides catalyzed by palladium and nickel species supported on alumina-based oxides - ScienceDirect
PLOS ONE: Supported Palladium Nanoparticles Synthesized by Living Plants as a Catalyst for Suzuki-Miyaura Reactions

Supported Palladium Nanoparticles that Catalyze Aminocarbonylation of Aryl Halides with Amines using Oxalic Acid as a Sustainable CO Source,Chemistry - A European Journal - X-MOL
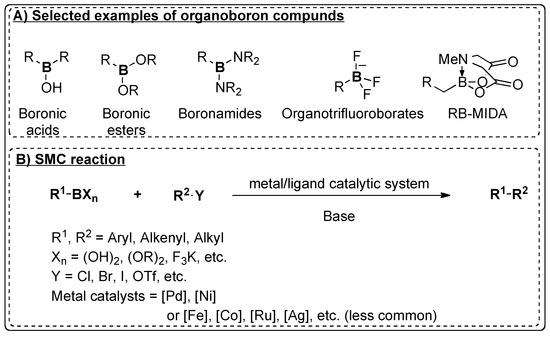
Catalysts | Free Full-Text | Recent Advances in Metal-Catalyzed Alkyl–Boron (C(sp3)–C(sp2)) Suzuki-Miyaura Cross-Couplings | HTML
![Palladium-catalyzed Suzuki cross-coupling of aryl halides with aryl boronic acids in the presence of glucosamine-based phosphines - [PDF Document] Palladium-catalyzed Suzuki cross-coupling of aryl halides with aryl boronic acids in the presence of glucosamine-based phosphines - [PDF Document]](https://demo.fdocuments.in/img/742x1000/reader018/reader/2020012214/575021491a28ab877e9f0ef3/r-2.jpg?t=1595669709)
Palladium-catalyzed Suzuki cross-coupling of aryl halides with aryl boronic acids in the presence of glucosamine-based phosphines - [PDF Document]