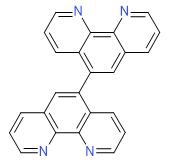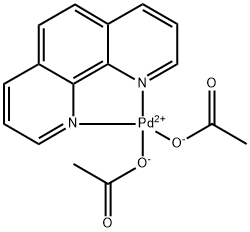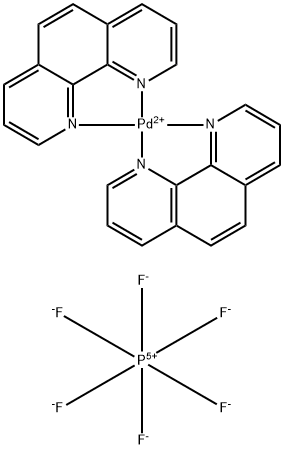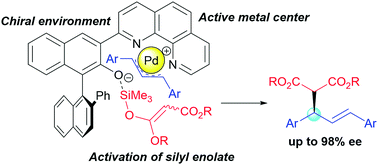
Design of bifunctional chiral phenanthroline ligand with Lewis basic site for palladium-catalyzed asymmetric allylic substitution - Chemical Communications (RSC Publishing)
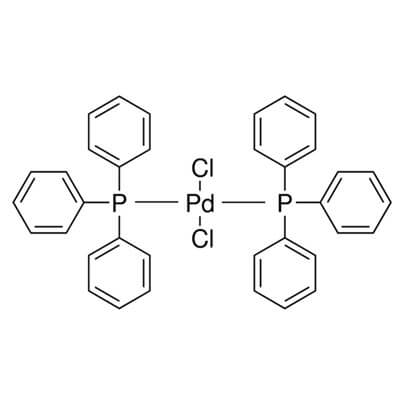
Experienced supplier of 13965-03-2,Pd(Pph3)2Cl2 PdCl2(PPh3)2,Bis (Triphenylphosphine)Palladium(II) Chloride

DNA interaction and anticancer evaluation of new palladium(II), platinum(II) and silver(I) complexes based on (Δ)- and (Λ)-1,2–bis-(1H-benzimidazol-2-yl)-1,2-ethanediol enantiomers - ScienceDirect
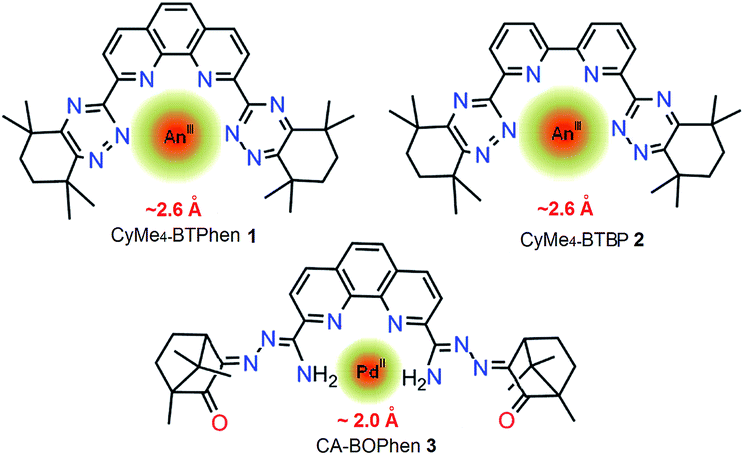
A phenanthroline-derived ligand and its complexation with Pd(ii): from ligand design, synthesis and Pd(ii) complexes structures to its application - RSC Advances (RSC Publishing)

Palladium(II) complexes of tridentate bis(benzazole) ligands: Structural, substitution kinetics, DNA interactions and cytotoxicity studies.,Journal of Inorganic Biochemistry - X-MOL
![1499115-56-8・Diphenyl Phenanthroline NNC Palladium [DPP-NNC Pd]・044-34351・040-34353[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation 1499115-56-8・Diphenyl Phenanthroline NNC Palladium [DPP-NNC Pd]・044-34351・040-34353[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation](https://labchem-wako.fujifilm.com/sc/01/1499115-56-8.png)
1499115-56-8・Diphenyl Phenanthroline NNC Palladium [DPP-NNC Pd]・044-34351・040-34353[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation
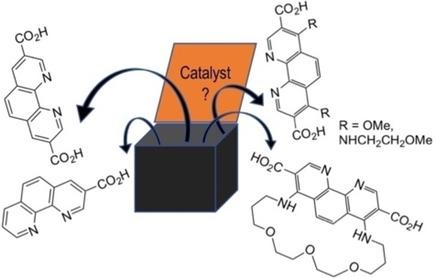
1,10‐Phenanthroline Carboxylic Acids for Preparation of Functionalized Metal‐Organic Frameworks - Asian J. Org. Chem. - X-MOL
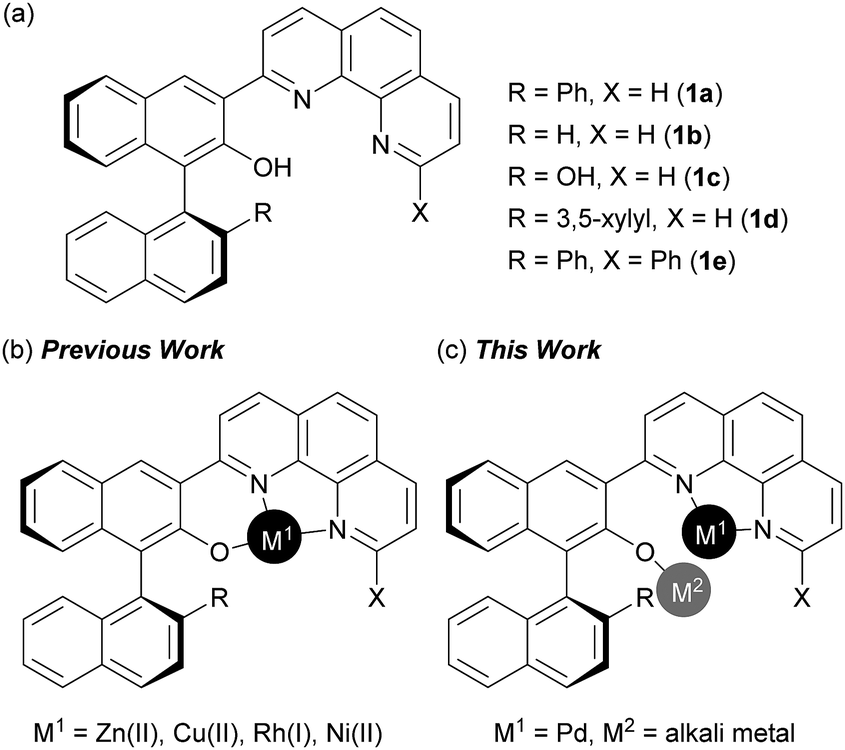
Design of bifunctional chiral phenanthroline ligand with Lewis basic site for palladium-catalyzed asymmetric allylic substitution - Chemical Communications (RSC Publishing) DOI:10.1039/C8CC00754C

Scheme 1 Synthesis of phenanthroline-and Pd(II)-phenanthroline-based... | Download Scientific Diagram
![PDF) Structure of a biologically active binuclear palladium complex in the compound (bis[(μ2-2-chloroethylammonium)(1,10-phenanthroline)-palladium(I)]) · tetranitrates · hydrate PDF) Structure of a biologically active binuclear palladium complex in the compound (bis[(μ2-2-chloroethylammonium)(1,10-phenanthroline)-palladium(I)]) · tetranitrates · hydrate](https://i1.rgstatic.net/publication/226391242_Structure_of_a_biologically_active_binuclear_palladium_complex_in_the_compound_bism2-2-chloroethylammonium110-phenanthroline-palladiumI_tetranitrates_hydrate/links/545137660cf24884d886fc4b/largepreview.png)
PDF) Structure of a biologically active binuclear palladium complex in the compound (bis[(μ2-2-chloroethylammonium)(1,10-phenanthroline)-palladium(I)]) · tetranitrates · hydrate

Bis(1,10-phenanthroline)palladium(II) Bis(hexafluorophosphate) 113173-22-1 | Tokyo Chemical Industry Co., Ltd.(APAC)

Design and synthesis of chiral 1,10-phenanthroline ligand, and application in palladium catalyzed asymmetric 1,4-addition reactions - ScienceDirect

Design and synthesis of chiral 1,10-phenanthroline ligand, and application in palladium catalyzed asymmetric 1,4-addition reactions - ScienceDirect

Scheme 1 Synthesis of phenanthroline-and Pd(II)-phenanthroline-based... | Download Scientific Diagram
![PDF) Structure of a biologically active binuclear palladium complex in the compound (bis[(μ2-2-chloroethylammonium)(1,10-phenanthroline)-palladium(I)]) · tetranitrates · hydrate PDF) Structure of a biologically active binuclear palladium complex in the compound (bis[(μ2-2-chloroethylammonium)(1,10-phenanthroline)-palladium(I)]) · tetranitrates · hydrate](https://www.researchgate.net/profile/Oxana_Magdysyuk/publication/226391242/figure/fig2/AS:393818487705601@1470905052104/Packing-of-the-nanocomplexes-in-the-structure-of-the-compound_Q320.jpg)
palladium(II)%20dimer%20bis(trifluoromethanesulfonate.jpg)


ruthenium(II)%20bis(hexafluorophosphate).jpg)