Palladium chemodosimeters based on change in optical properties. (a)... | Download Scientific Diagram

Palladium‐Catalyzed Oxygenative Cross‐Coupling of Ynamides and Benzyl Bromides by Carbene Migratory Insertion - Gao - 2018 - Angewandte Chemie International Edition - Wiley Online Library

TryR inhibitors derived from palladium nitrofurylthiosemicarbazone and... | Download Scientific Diagram

The scope and mechanism of palladium-catalysed Markovnikov alkoxycarbonylation of alkenes | Semantic Scholar
![PDF] Palladium-mediated annulation of vinyl aziridines with Michael acceptors: stereocontrolled synthesis of substituted pyrrolidines and its application in a formal synthesis of (-)-α-kainic acid. | Semantic Scholar PDF] Palladium-mediated annulation of vinyl aziridines with Michael acceptors: stereocontrolled synthesis of substituted pyrrolidines and its application in a formal synthesis of (-)-α-kainic acid. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/12fed18ef8b4cf44ff31fec1138c3dc71af93c47/2-Table2-1.png)
PDF] Palladium-mediated annulation of vinyl aziridines with Michael acceptors: stereocontrolled synthesis of substituted pyrrolidines and its application in a formal synthesis of (-)-α-kainic acid. | Semantic Scholar

Palladium-Catalyzed Oxidative Cross-Coupling of Conjugated Enynones with Allylarenes: Synthesis of Furyl-Substituted 1,3-Dienes - J. Org. Chem. - X-MOL
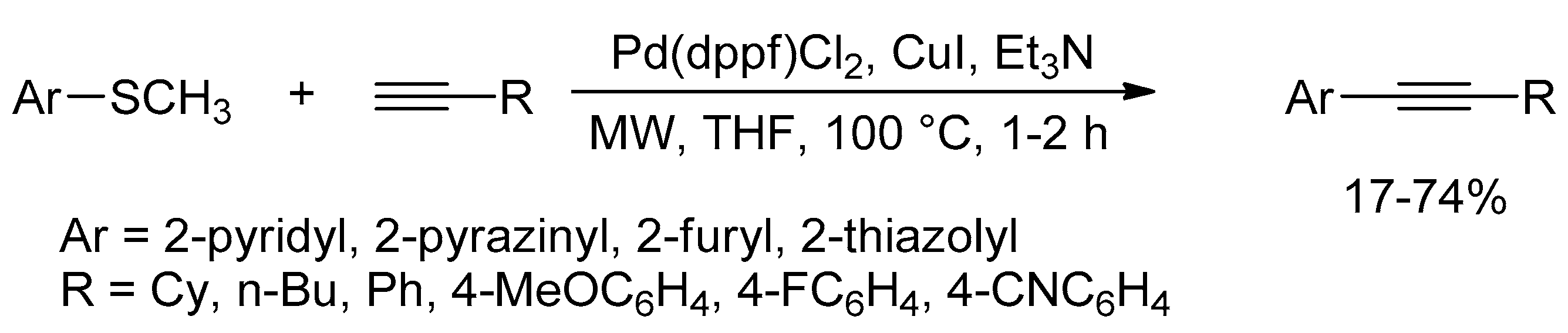
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond
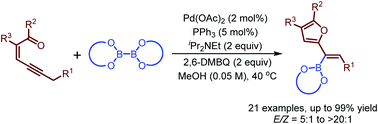
Palladium-catalyzed oxidative borylation of conjugated enynones through carbene migratory insertion: synthesis of furyl-substituted alkenylboronates - Chemical Communications (RSC Publishing)
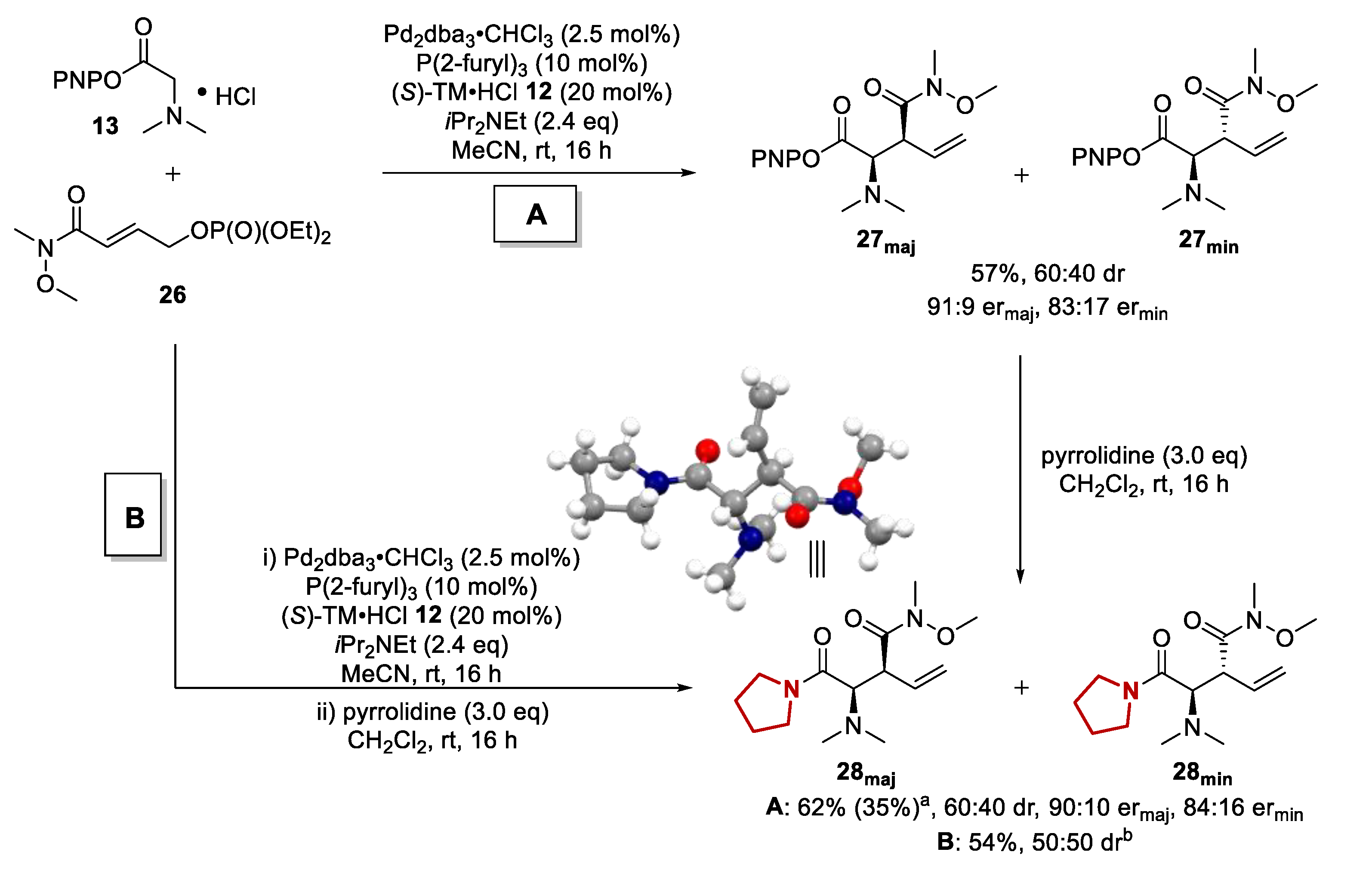
Molecules | Free Full-Text | Exploring the Scope of Tandem Palladium and Isothiourea Relay Catalysis for the Synthesis of α-Amino Acid Derivatives
![5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation 5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation](https://labchem-wako.fujifilm.com/sc/01/5518-52-5.png)
5518-52-5・Tri(2-furyl)phosphine・202-18631・208-18633[Detail Information] | [Synthesis & Materials] |Laboratory Chemicals-FUJIFILM Wako Chemicals U.S.A. Corporation

Use of a bulky phosphine of weak σ-donicity with palladium as a versatile and highly-active catalytic system: allylation and arylation coupling reactions at 10−1–10−4 mol% catalyst loadings of ferrocenyl bis(difurylphosphine)/Pd - ScienceDirect

Palladium labile precursors of 5FU (1) and gemcitabine (2). (b) FUdR... | Download Scientific Diagram

Noneffervescent Method for Catalysis-Based Palladium Detection with Color or Fluorescence,ACS Sensors - X-MOL

A Palladium‐Catalyzed Dehydrogenative Diamination of Terminal Olefins - Wang - 2008 - Angewandte Chemie International Edition - Wiley Online Library

Dual catalytic processes employing isothiourea and palladium catalysts:... | Download Scientific Diagram
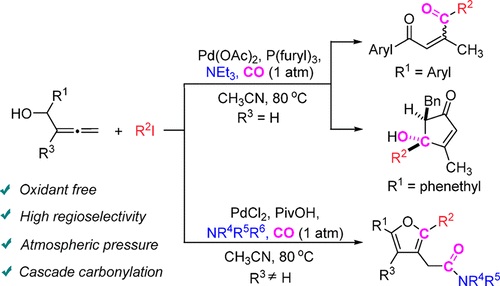
Tunable Synthesis of 2-Ene-1,4-diones, 4-Hydroxycyclopent-2-en-1-ones, and 2-(Furan-3-yl)acetamides via Palladium-Catalyzed Cascade Reactions of Allenols - J. Org. Chem. - X-MOL