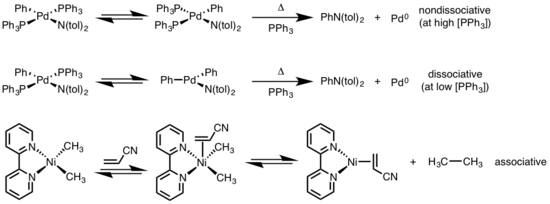
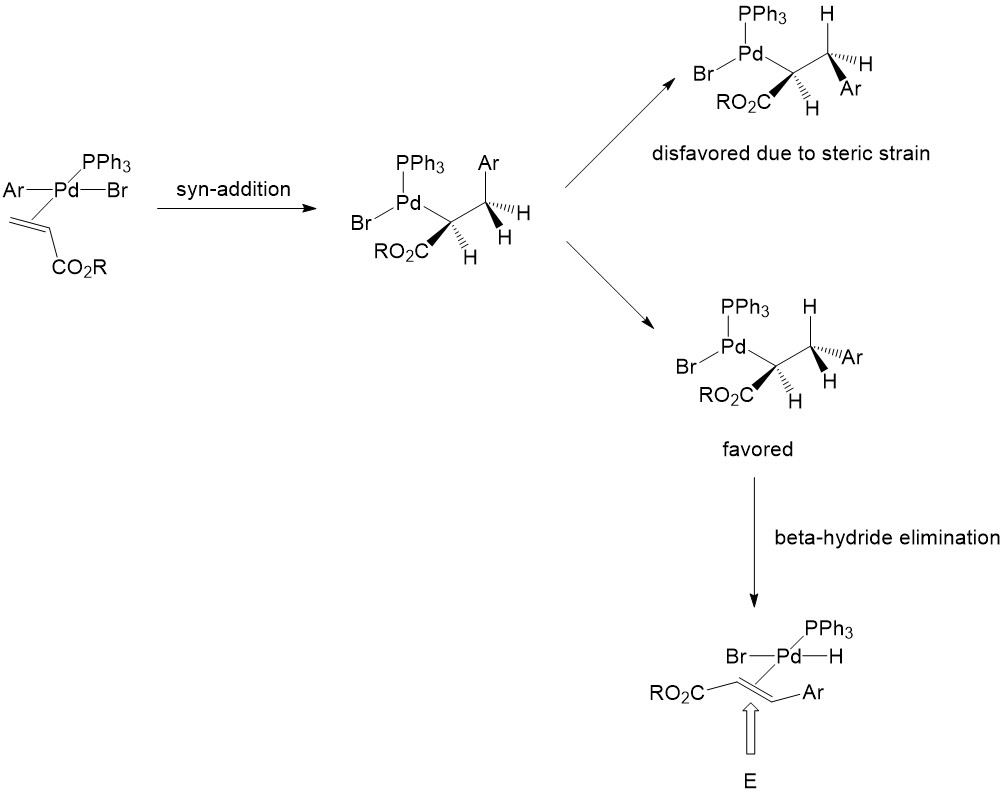
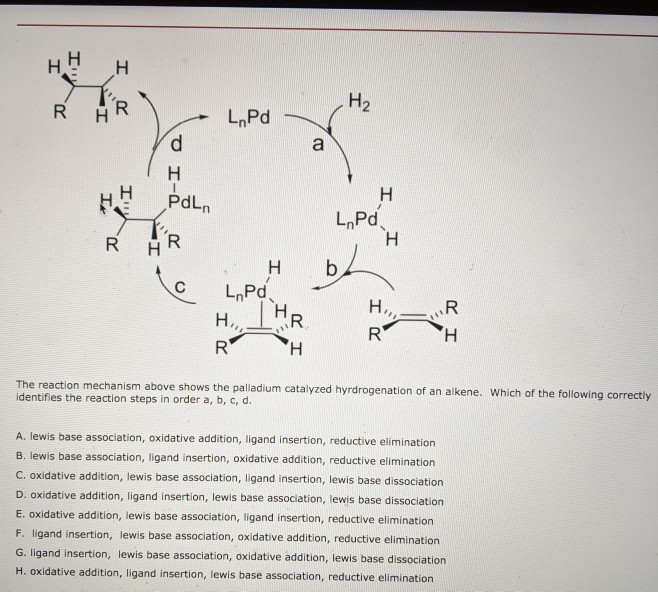
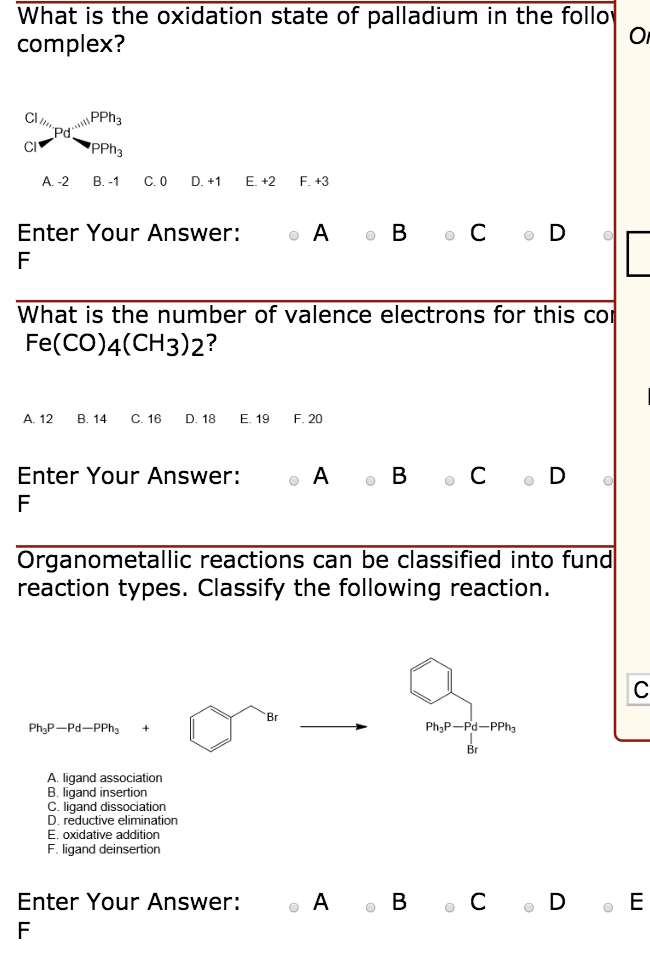
Mechanistic experiments support the proposed β-elimination mechanism... | Download Scientific Diagram

Activation of diverse carbon–heteroatom and carbon–carbon bonds via palladium( ii )-catalysed β-X elimination | Nature Chemistry
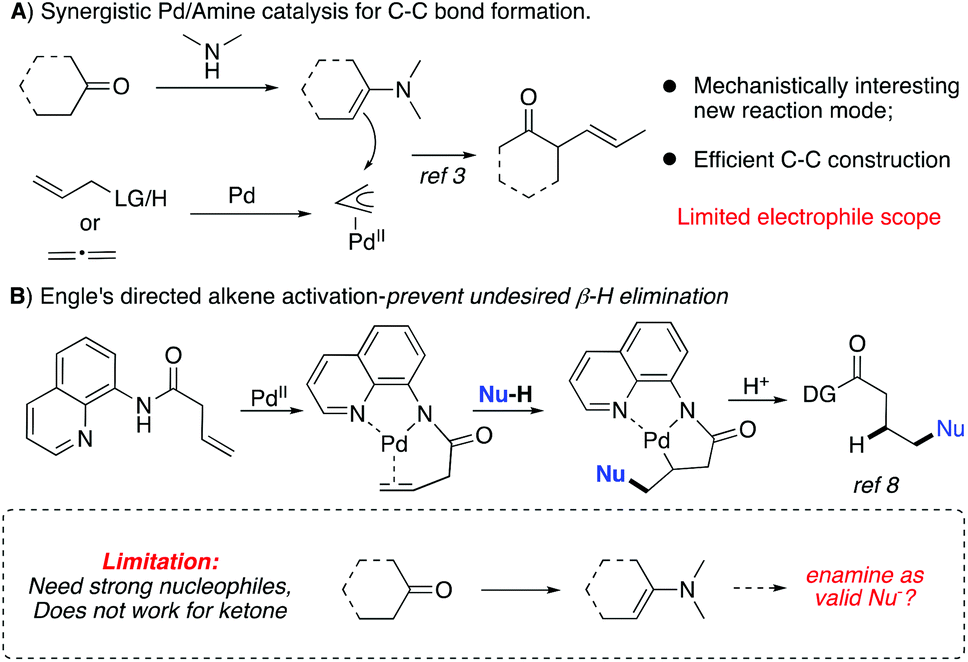
Synergistic palladium/enamine catalysis for asymmetric hydrocarbon functionalization of unactivated alkenes with ketones - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C9OB01165J

Figure 1 from Chemical remodeling of cell-surface sialic acids through a palladium-triggered bioorthogonal elimination reaction. | Semantic Scholar
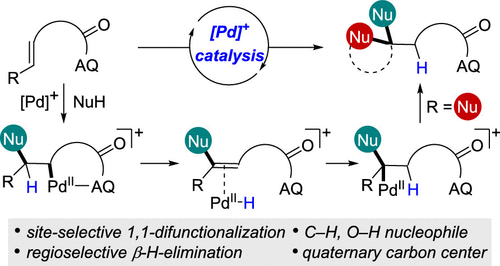
Site-Selective 1,1-Difunctionalization of Unactivated Alkenes Enabled by Cationic Palladium Catalysis. - J. Am. Chem. Soc. - X-MOL

From Benzofurans to Indoles: Palladium‐Catalyzed Reductive Ring‐Opening and Closure via β‐Phenoxide Elimination - Perego - 2019 - Advanced Synthesis & Catalysis - Wiley Online Library
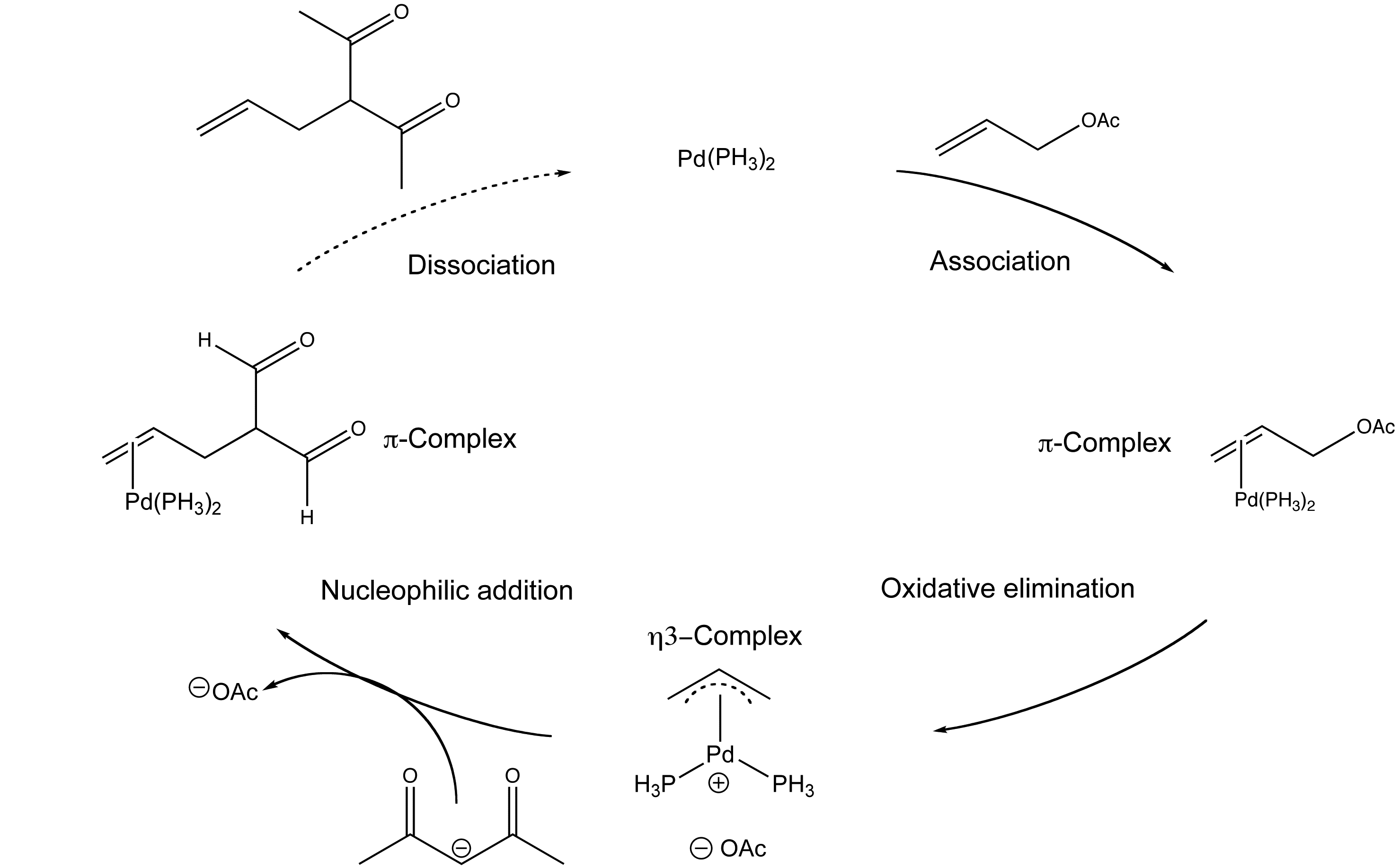
Organopalladium Chemistry - Palladium-catalysed nucleophilic allylic substitution of functionalised compounds
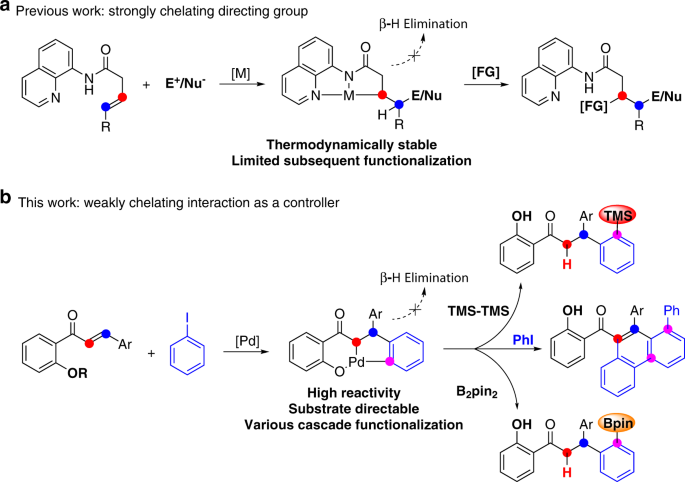
Quadruple C-H activation coupled to hydrofunctionalization and C-H silylation/borylation enabled by weakly coordinated palladium catalyst | Nature Communications
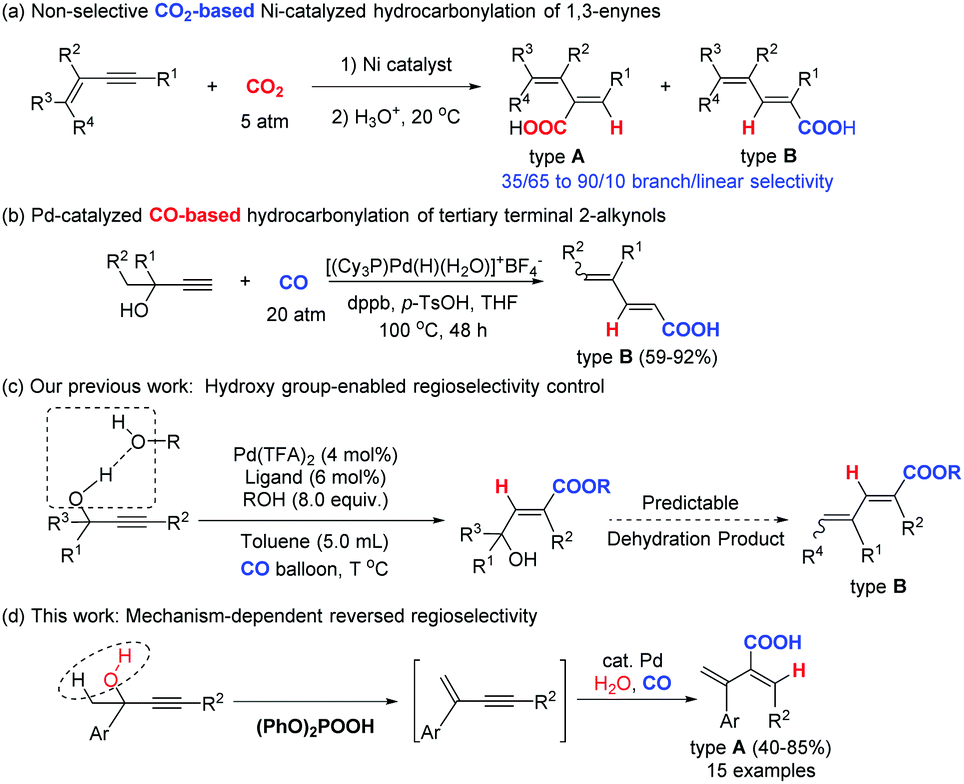
Palladium catalyzed regioselective elimination–hydrocarbonylation of propargylic alcohols - Chemical Communications (RSC Publishing) DOI:10.1039/C9CC03262B
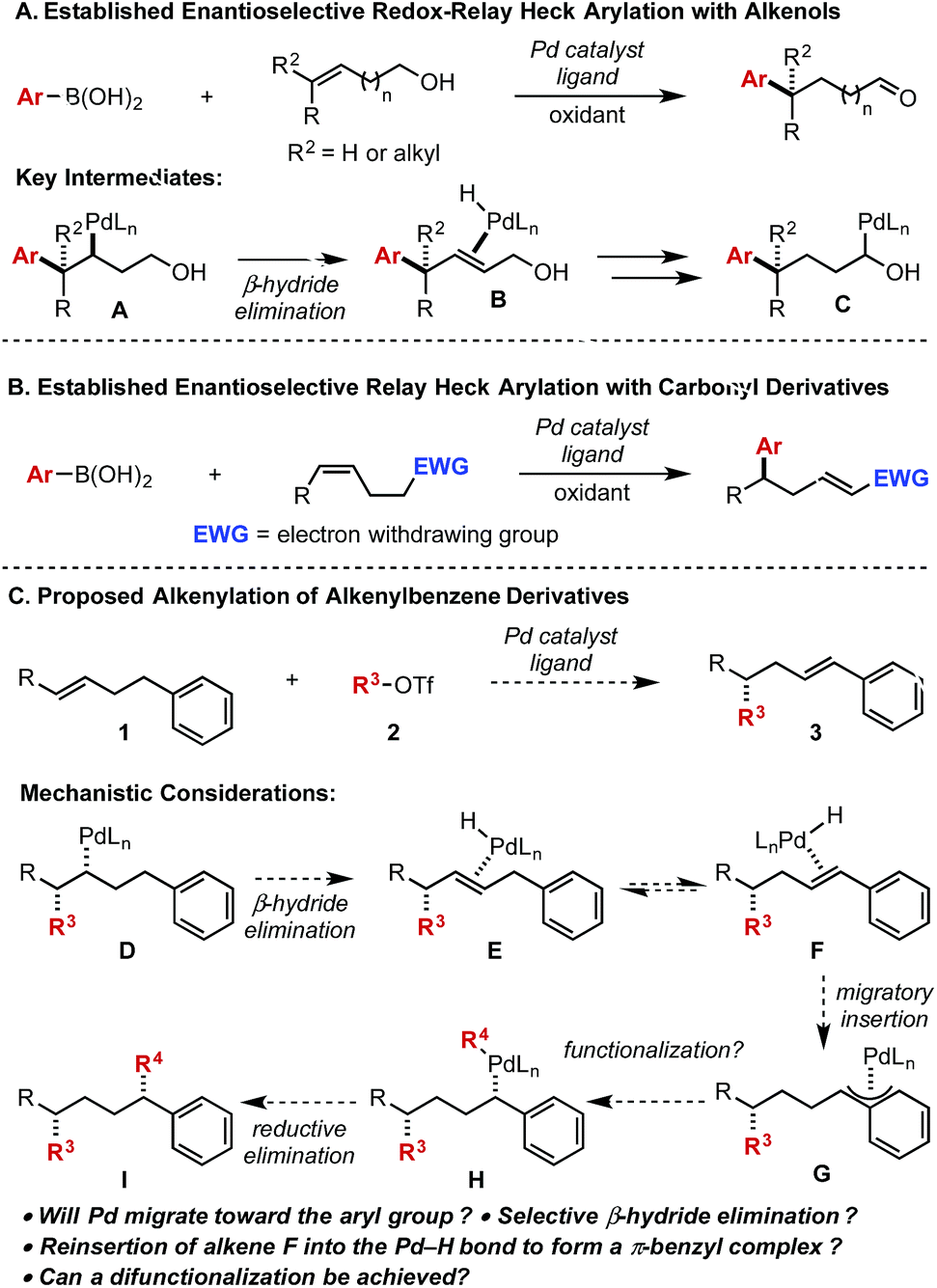
Palladium-catalyzed enantioselective alkenylation of alkenylbenzene derivatives - Chemical Science (RSC Publishing)
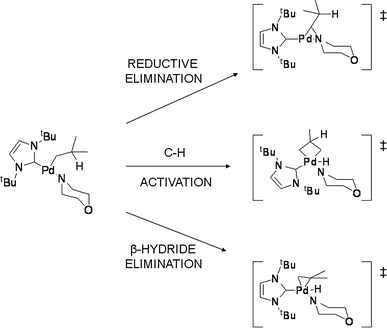
DFT studies of reductive elimination, C–H activation and β-hydride elimination in alkyl and aryl palladium amine complexes | SpringerLink
![Constructing chiral bicyclo[3.2.1]octanes via palladium-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes | Nature Communications Constructing chiral bicyclo[3.2.1]octanes via palladium-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes | Nature Communications](https://media.springernature.com/lw685/springer-static/image/art%3A10.1038%2Fs41467-020-16221-9/MediaObjects/41467_2020_16221_Fig1_HTML.png)
Constructing chiral bicyclo[3.2.1]octanes via palladium-catalyzed asymmetric tandem Heck/carbonylation desymmetrization of cyclopentenes | Nature Communications

Activation of diverse carbon–heteroatom and carbon–carbon bonds via palladium( ii )-catalysed β-X elimination | Nature Chemistry
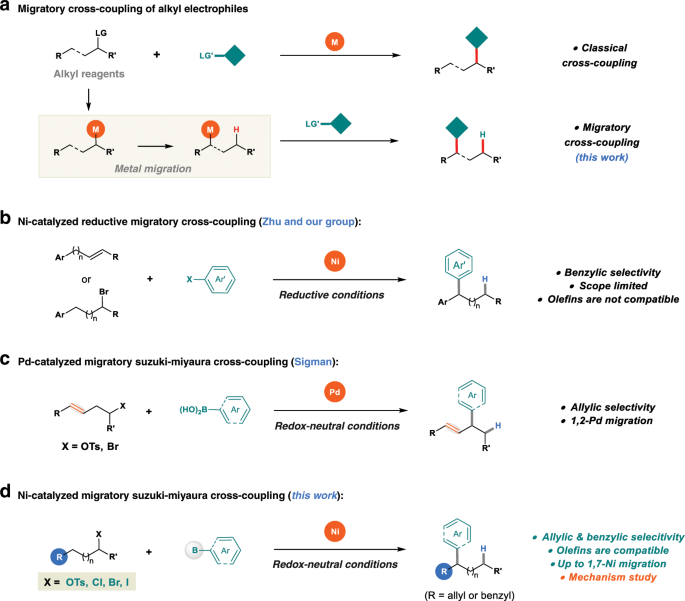
Reaction scope and mechanistic insights of nickel-catalyzed migratory Suzuki–Miyaura cross-coupling | Nature Communications
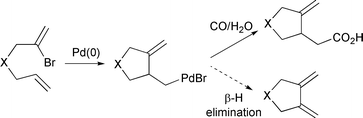
A palladium catalysed cyclisation–carbonylation of bromodienes: control in carbonylation over facile β-hydride elimination - Chemical Communications (RSC Publishing)

Activation of diverse carbon–heteroatom and carbon–carbon bonds via palladium( ii )-catalysed β-X elimination | Nature Chemistry
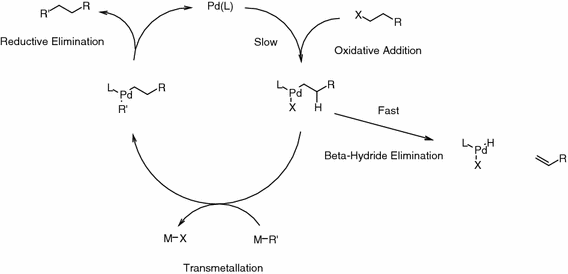
DFT studies of reductive elimination, C–H activation and β-hydride elimination in alkyl and aryl palladium amine complexes | SpringerLink